This post documents our work using urine as a primary nutrient in hydroponic systems. It is a part of a series of blog posts exploring the historical and modern applications for human urine. Part 1 is available here. Part 2 is available here.
Three years ago, in the early spring, I bought two used, food-grade plastic 55 gallon drums, cleaned them out, sawed one in half and put the other in the back corner of the insulated shipping container that was then serving as our black soldier fly larvae production room. I bought and installed a small 120 volt aquarium pump and filled the large drum with well water. I carefully filled the remaining half-drum with washed walnut-shell biochar and arranged it to sit above my larger water-filled drum on a welded stand. Then I carefully measured 100 mL of my own urine and poured it in.
These were the first steps in building out a small experimental aquaponics system, the purpose of which was to help us answer a few questions while exploring first hand if it would be appropriate for us to scale up:
- First, How would our walnut shell biochar perform as an alternative to conventional hydroponic grow media?
- Could we build and maintain a functioning system on a very small budget using mostly salvage materials?
- And last, what would it take to maintain low expenses by entirely eliminating the need for imported fish feed?
Think of aquaponics as a pairing of conventional aquaculture with hydroponic growing. In these recirculating systems, fish waste is made available as plant nutrients by means of nitrifying bacteria, where then plants uptake these nutrients as they grow, in turn, cleaning the water for the fish. Farm-raised fish boast an incredibly efficient feed conversion ratio, and aquaponics takes this incredible nutrient efficiency a step further by growing vegetables with what would otherwise be wasted, either filtered or drained away. These can be remarkably elegant systems – once dialed in, aquaponically grown vegetables can be incredibly productive and nutritionally efficient. Furthermore, aquaponic systems show potential for hyper-local organic vegetable and protein production on degraded lands, dense urban areas, or even indoors.
Those that have tried know that problem-free aquaponics is easier said than done. Conventional gardening problems persist alongside the challenges of aquaculture. Ideal water characteristics for fish don’t always match ideal conditions for vegetable growth. Fish waste is a mostly-balanced fertilizer, but it’s not quite complete – micronutrient supplementation is still necessary. Mechanical problems arise too: filters clog, drains overflow, sometimes the power goes out. And though profitable commercial aquaponics is possible, these are systems that must be continually monitored and maintained by qualified technicians.
Given this was anyone on the crew’s first time with any aquaculture, hydroponic growing, or even any kind of indoor growing for that matter, it goes without saying we had our share of challenges. Looking back, it’s possible we weren’t feeding our Tilapia fry enough, though it’s equally as likely we were battling some kind of disease pressure. Ammonia levels were always in check, but nitrate levels in the water were never adequate and it’s certain we hadn’t provided adequate light for our kale. In hindsight, sharing the heated space with the black soldier flies wasn’t helpful either. By winter, gas exchange was low and humidity remained so high that feed pellets were molding over the weekend in our DIY automatic feeder. We hadn’t even begun to breach the issue of eliminating purchased feed before losing the last of our tilapia fingerlings, at no more than 6 months old, by December 2018.
This early foray into aquaponics was not a complete failure. For a very low cost we had built out the hardware of a functioning system and our biochar media had performed very well. Though removal of dead fish from the system was a humbling experience; it is a reminder of an obvious point: that the most successful farming systems are the ones that can be appropriately managed. One thing was abundantly clear: to be successful in any future hydroponic experimentation we were going to have to simplify.
At the time, I dismantled the system, and would not return to it for another two years, during which my exploration into the fertilizing applications for human urine would eventually guide me back to the world of soil-less growing. It was then, near the start of the covid pandemic, that I first came across the term anthroponics used to describe a method of hydroponic growing where human urine was the primary nutrient source. As a form of organic hydroponic growing anthroponics combines elements of both conventional hydroponic cultivation and aquaponics.
I’ll use the remainder of this post to share my work with anthroponics, but first, to get a better sense of how these systems work, it’s helpful to understand the basics of aquaponics.
Aquaponics 101
In aquaponics systems, the fish waste to nutrient transformation takes place in oxygenated water by means of nitrifying bacteria. First, fish excrete ammonia through their gills. Additional ammonia and phosphorus enter the water via fish urine and mineralizing detritus. If left unchecked, too much ammonia poisons the fish – this is where nitrifying bacteria come in. This process, called nitrification, takes place on a biofilter, where bacteria convert poisonous ammonia first to nitrites, and then to plant-available nitrates. It’s these nitrates, along with phosphorus and minerals from decaying detritus that make up the array of plant nutrients.
Through a process known as cycling, a well-functioning biofilter with robust populations of nitrifying bacteria must first be established in new systems before introducing meaningful quantities of fish. Cycling can be accomplished in two broadly different ways:
- Gradually add fish over weeks or months. Nitrifying bacteria is ubiquitous and will make its way in, but it will take a long time to accumulate the nitrates needed to sustain vegetable production. Plants must be slowly added too.
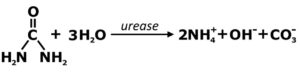
- Inoculate with water/media from a healthy, functioning system or use commercially-available cultured bacteria, and then introduce your own ammonia. Urine is a perfectly acceptable, natural and safe source of ammonia. “Fish-less cycling” with added ammonia is an all around faster way to establish a biofilter.
The same rules apply for fresh-water home aquariums too. Healthy fish life is supported by nitrifying bacteria. The key difference here is that nitrates are typically adsorbed in disposable activated carbon filters rather than made available as a plant nutrient and continuously removed as plants grow. Recirculating aquaponics systems are about striking balance: the inputs must coincide with outputs. It’s important to realize that even though the plants are essentially growing on nutrients from fish waste, these nutrients aren’t free. Fish feed is the input, i.e. the source of fertility, and conventional fish feeds are almost synonymous with unsustainability – conjuring up images of depleted fisheries and thousands of acres of monocropped soybeans. And while there are sustainable high-protein feed options, many of which can be farmed on site: black soldier fly larvae, duckweed, etc., these are farming systems in and of themselves that require inputs and support.
Ironically, it was the prospect of a low-cost and limited-waste source of protein that drove my initial interest in aquaponics. It was these early failures that led to an alternative hydroponic cultivation method that still utilized waste and was significantly easier to manage.
Aquaponics Without the Fish
Recall that a biofilter can be established by adding ammonia in the form of urine. One could think of anthroponics as an alternative, where instead of tapering off urine and relying on fish feed/waste, nitrate production is simply maintained by the regular addition of urine.
Typical human urine is about 95% water; the nitrogenous compound urea makes up about half of the remaining 5%. In time, by way of the ubiquitous enzyme urease, the urea present in urine will convert to ammonium, hydroxide and carbonate ions. When urine is stored in closed containers for up to a few weeks, this process occurs naturally – it’s a good thing – the elevated pH helps mitigate possible bacterial contamination and the ammonium mimics fish waste and itself kicks off the nitrification process. Extended storage is not the only method to ensure sanitation of urine fertilizer, but is by far the simplest.
Anthroponics shares many of the advantages of both aquaponics and conventional hydroponic growing. Like all hydroponic growing, water demand is drastically lower than conventional agriculture. Growing conditions – environment, nutrient balance, etc – are highly controllable; they’re good for experimentation, and there’s potential for high food production capacity even in desertified or urban settings. As in aquaponics systems, there is no environmental cost of resource intensive, petroleum-based chemical fertilizers. It goes without saying too that unlike conventional fertilizers, urine is not subject to the whims of the market. Anthroponics systems are biologically based too, and by extension of the resulting food-web, when compared to conventional hydroponic culture they are likely more robust against pest and disease pressures.
Anthroponics systems have certain advantages compared directly to aquaponics systems. For starters, there is no feed requirement associated with aquaponics. With no fish to manage there’s no solid waste, and therefore no need for additional filters, tanks and hardware. Temperature and pH still play a role in effective nitrification and plant health, but here they are no longer critical life and death factors. There is no question that anthroponics systems are much easier to maintain.
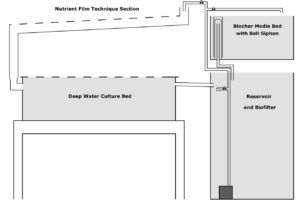
Our pilot anthroponics system built off our original 50 gallon drum tank arrangement. In addition to the full size drum reservoir and half drum biochar-media bed, I added a 7 foot section of 3 inch PVC as NFT channel that flows around into a single 100 gallon IBC tote bottom as a DWC raft bed. The outflow from the raft bed returns directly back to the reservoir. Our small aquarium pump is located in the lowest point of the system and by way of simple manually operated ball valves, flow can be directed to one of three paths: directly into the media bed, through the NFT and DWC route, or as a means for both flow control and oxygenation, directly back over the biofilter/reservoir.

Our initial indoor arrangement, with various lettuces in both NFT and DWC sections. Celery is in the biochar media bed on the right.
Biofiltration
Biofiltration largely refers to the conversion of ammonia/ammonium to plant-available nitrates by means of nitrifying bacteria. Nitrifying bacteria thrive in neutral-warm, highly oxygenated water where they cling to surfaces and form biofilms that act as sticky filters, trapping suspended solids like bits of stray plant material and grow media. A typical biofilter in a large aquaponics system commonly looks like a dedicated tank with vigorously aerated suspended plastic media. A biofilter can be more basic too; our biochar media functions as a biofilter. In the introductory anthroponix tutorial from Hong Kong’s Dim Sum Labs, it’s the rooting media itself that functions as a biofilter.
In addition to the 20 gallons of walnut biochar media as filter, we employ a (in my opinion) rather clever and low cost solution for creating even more surface area: HDPE plastic flakes. Through our previous work with on-farm plastic recycling we had amassed plenty of used gallon-size milk jugs from area restaurants and coffee shops. Food-grade HDPE is widely considered the most benign plastics – BPA, plasticizers, etc. are not a concern here. In our typical process these jugs would be cleaned and shredded into flakes before injection molding or extrusion. Here, about 1kg (the equivalent of 16 whole jugs) of these shredded flakes are simply loosely packed into nylon mesh paint strainer bags. By my estimation, each jug provides 450in2 of additional surface area. By adding 5 flake bags (of 16 jugs each) in the reservoir, we add an additional 250ft2 of surface area.
First Harvests
By January of this year we had a fully functioning and cycled anthroponics system, installed under salvaged T5 fluorescent lights hung from the ceiling in the back of our insulated shipping container. By now our goals had changed, humbled by initial failures of my foray into aquaponics, I now set out to find answers to more simple, albeit meandering questions: Could we demonstrate the long-term feasibility of urine as a fertilizer in recirculating hydroponics? What kind of problems would present themselves after multiple harvest cycles?
I first germinated an array of romaine and loose-leaf lettuces in small net pots filled with composted pine bark media; these were transplanted to the NFT and DWC as soon as the first true leaves appeared. Celery shoots were transplanted from soil to the media bed at the same time and over the succeeding two weeks I added 2.5 liters of regular urine to reach a 200:1 water-to-urine ratio.
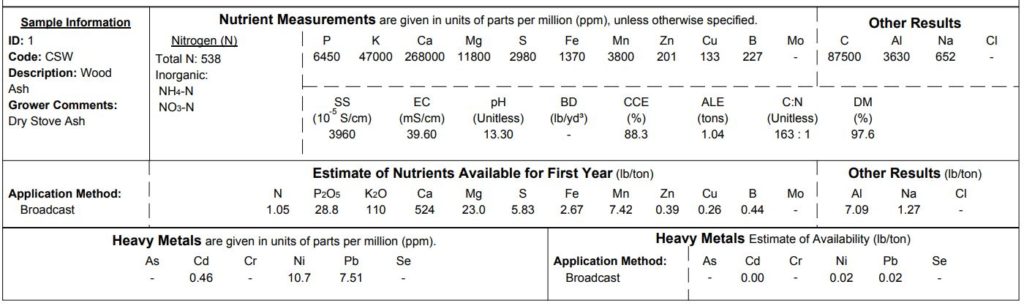
As a plant fertilizer, urine is relatively high in nitrogen and phosphorus and is most closely balanced for growing leafy greens. Potassium is present, but not at balanced levels for growing vegetables – even low nutrient crops like lettuce will need supplemental potassium. Wood ashes can be a potent source of readily soluble potassium, along with calcium and a host of other micronutrients, but these too are Excesses can be a problem too: because this is a closed loop recirculating system where salts won’t readily leach from the soil, sodium and chloride ions from the urine, along with other micronutrients from the wood ash may eventually reach toxic levels.
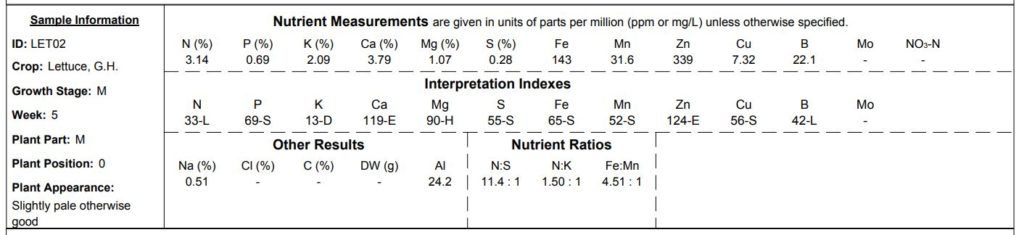
Knowing that imbalances could quickly manifest, I added celery in the system specifically to help remove sodium; because of its brackish origins I figured celery would thrive here. Lettuces, on the other hand, are known to be more sensitive to high sodium concentrations. Nonetheless, without accurate analysis, at this point I was relying solely on external indicators of imbalances – leaf malnormaties, odd flavors, stunted growth, etc. In each case it wasn’t long before nutrient deficiencies began to manifest, first on the leaves of the romaine lettuce and then later on the celery.

Pale and curling from indoor-grown romaine lettuce leaves. Samples of this crop were sent to the NCDA lab for analysis
Nutrient Balancing
The balance of nutrients in urine varies considerably based on diet and time of day collected. A high-protein diet yields higher nitrogen. Hydration level is obviously an important factor. The Rich Earth Institute in Brattleboro Vermont, USA, reports an NPK value of 0.6-0.1-0.2 in urine collected from their community urine donation program. Urine collected in morning has long been assumed to be more potent. Looking to validate this assumption I had a composite sample of my morning-urine sent off for analysis:
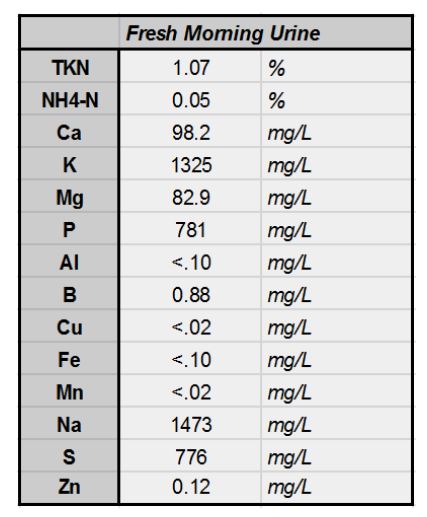
Nutrient Analysis of my own morning-urine. NPK: 1.0-.07-.13. Note the significant quantities of sodium.
Note the substantially higher nitrogen concentration in my morning urine. Also, take note of the actual lower concentration of phosphorus and potassium. Should I expect my daytime urine to have higher phosphorus and potassium content? Is this an indication these minerals are lacking in my diet? It may not be as useful to keep morning and daytime urine separate. Nonetheless, this morning sample is my known composition and barring any radical diet changes, it’s what I’ll plan to use in the anthroponics system moving forward.
Urine alone will not be a balanced fertilizer for growing much of anything. Even though lettuce and other leafy greens prefer a relatively high balance of nitrogen compared to other nutrients, I’ll still need to include additional potassium, phosphorus and other minerals. Wood ash, another ‘waste’ product I’ve talked a lot about before, does a very nice job bringing us closer to a balanced nutrient solution. Like urine, there’s substantial variation in the nutrient composition of wood ash. A composite sample of a prior year’s wood stove ash was sent off for analysis:
I sought out and compared three different guidelines for an ideal nutrient solution for growing hydroponic lettuce: from this Cornell publication, guidelines from the NCDA, and from J.Benton Jones’ Practical Guide for the Soilless Grower (quite affordable when only rented for a month on the kindle app) . Using these guidelines, along with the nutrient values from my urine and wood ash analysis, I built a simple spreadsheet and from there was able to identify additional nutrient deficiencies and design a custom ‘mineral blend’ that would accompany and balance the nutrient profile of my urine.
This custom mineral blend includes primarily wood ash and gypsum, along with a small amount of magnesium oxide some of my (wood ash-sourced) potassium carbonate. I blended together all of these ingredients in substantial quantities – enough to last me for at least another year of growing. Now, for each 200 ml dose of morning urine, I’ll add just shy of 1 tablespoon of mineral mix. I’ll also add 30mL of chelated iron for each full liter of urine. The recipe that follows accompanies 1 liter of my morning-urine:
30 grams Wood Ash (mixed Appalachian hardwood, stove ash)
4 grams Gypsum (calcium sulfate)
4 grams magnesium oxide
10 grams potassium carbonate
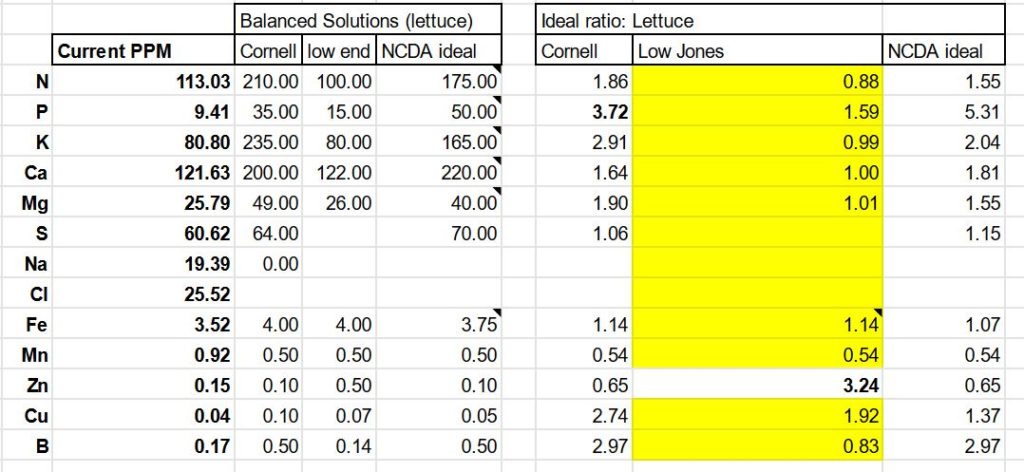
Determining proper mineral blend with a custom spreadsheet: The closer the numbers are to 1, the more perfect the ratio of nutrients is to the stated ideal.
Spring Harvests
Now with some confidence that I had control of the nutrient solution I added a few more varieties of lettuce transplants to the DWC raft and NFT sections. At the same time, now early February, I introduced a few swiss chard plants as well. Swiss chard is a known sodium-hungry crop with similar high nitrogen feeding requirements. For what it’s worth, I found germination to be quite satisfactory when seeded directly in 2” net pots filled with aged pine bark.

Loose leaf lettuce varieties – oak leaf lettuce in particular – both presented and tasted the best overall
In this period starting from when I switched to a complete mineral blend on 2/17 to 4/9 I added only 6.4 liters of urine and harvested 3 kg of Oak Leaf lettuce, 9.8 kg of Giant Caesar lettuce, and 6.2 kg of Swiss Chard leaves. Additionally there was some celery, but this was not accounted for by weight. There is significant overlap here with some plantings done prior to 2/17, and the nutrient solution had not been completely changed out yet at this point – these figures should be taken cautiously, nonetheless it’s helpful to see what kind of productivity one could expect.
Summer Harvests
By May I was convinced I had the nutrient solution more or less dialed in and temperatures had warmed enough so that I could move the entire operation outside. This gave me a great advantage, not just by substituting energy-intensive artificial lights for the real thing, but also the gentle wind and free air gave much improved vigor to all subsequent plantings. In hindsight, my hunch it’s likely that these initial malnormatles with the lettuces were due in large part to irregular calcium uptake, caused in large part to a confluence of negative environmental factors in the indoor grow room: high humidity, light was possibly TOO intense at times and not completely full spectrum, and little gas/air exchange.
Upon moving the system hardware outside I refilled the system with fresh well water and dosed urine and corresponding minerals at the initial rate of 1 liter urine per 200 liters of water. Additionally, I added soluble humates and a small amount (about 2 cups) vermicompost extract to the reservoir. From this point forward I would strive to incrementally build up to a consistent nutrient level through routine dosing of urine and minerals, while consistently monitoring EC, maintained at a conservative 700 us/cm.
For the next round of plantings in the media bed I swapped out celery in favor of two other swiss chard varieties. When inside, in the DWC raft bed, I found the chard quickly grew large enough to easily fall over in net pots; I found Swiss chard much better suited for planting in the biochar media bed. Along with lettuces in the NFT section, these chard plants proved very productive right up until the end of October this year.
When exclusively growing lettuces indoors – and I’m sure this won’t be surprising to anyone – I could not find anyone outside of my family willing to eat the lettuces I was growing. What I did find a bit surprising however, was that their prejudice was less about urine-as-fertilizer, and more about hydroponic growing in general. I’m much too green to take this debate head on – but I would encourage those who doubt that nutrient-dense food can be grown hydroponically using organic inputs to at least first read about others’ work in ‘bioponics’, or at least check out the conversation going on at the Organic Hydroponics facebook group before settling on any opinions.
That said, 27 heads of lettuce is still too much for my family. Having moved outside for the summer, I chose to clear out the space on my DWC raft and make room for experimentation with Japanese indigo. Persicaria tinctoria is known to propagate easily in water and it thrives in summer heat. In a previous post I wrote about my success with urine fertilization of Japanese Indigo in my gardens. Now I wanted to compare the growth rate and pigment yield of my anthroponic-grown indigo to my prior year’s soil grown plants.
Nitrification is much faster with warmer water. This was clearly seen as temperatures continued to rise as the summer progressed – I was no longer seeing the pH fluctuations after dosing with high pH urine. I found a rhythm here – for the most part, pH held steady around 6.5 and EC was consistent around 650 us/cm with regular dosing of around 1 Liter urine / week.
While warmer water speeds up nitrification and encourages growth, there are reasons to prevent the nutrient solution from getting too hot. Warmer water holds less oxygen and potential pathogen proliferation is more likely in higher temps. Cool season plants will prefer cooler water: this was especially evident later in the season as the lettuces and Swiss chard became increasingly bitter. However, the Japanese Indigo was thriving in the warm summer temperatures.
On May 17, 20 Japanese Indigo starts in 2” net pots were transplanted to the DWC raft bed. This was immediately after the cotyledon stage, when the first true leaves appeared. Eight weeks later, in mid-July, the plants were over 18” high and ready for the first harvest. This first harvest yielded 12.5 lbs of fresh plant biomass. Subsequent harvests were ready in just 3 weeks’ intervals: on 8/6 I harvested 9.5 lbs, on 8/31 I harvested 10.25 lbs. I found this to be an extremely fast growth-rate. What is not clear yet is the quality and yield of the indigo pigment – when extracting pigment from this ‘anthroponically’ grown indigo, I found the fermentation to be incredibly fast. Upon floculation after only 36 hours’ fermentation time I had a brown, presumably organic-rich supernatant. Compared to my soil-grown indigo, these plants are tender; I suspect I had already ‘over-fermented’ each batch in a very short time.
Where to go from here?
Anthroponics/peeponics/bioponics – whatever you choose to call this method of hydroponic growing with urine – is not without its challenges. Urine must be collected far enough in advance to ensure adequate sanitation. There is the obvious public perception problem. Regarding recirculating systems, there is the persistent issue of variability of nutrient profiles among urine and wood ash. Given enough time it seems inevitable that some problematic nutrient imbalances will occur.
As I write this, I’ve got the system set up again for indoor growing under dappled artificial light. For the winter, I’ve switched to growing two different wild sourced duckweeds: Lemna minor and Spirodela polyrhiza. In many ways duckweed is the perfect crop for an anthroponic system; duckweeds thrive in slow moving nutrient-rich water; they are one of nature’s filters for nutrient polluted waterways. Duckweed is well known as a fast growing, protein rich fish and poultry feed – though I suspect many may not be aware that the protein content of duckweed is closely linked to the concentration of available nitrogen in the water. Late summer 2021 trials show that Spirodela polyrhiza, originally sourced from the edges of our irrigation pond, is almost three times as protein-rich when cultured in my anthroponics system. Indoor growing has not yet proven to match the productivity of late summer 2021. Suffice to say, I’m looking forward to setting up a scaled-up version of the duckweed farm for next summer.

Wild-sourced duckweed on right – 15.6% crude protein. Anthroponically-grown duckweed on left – 39.8% crude protein.
In many ways, growing high protein fodder may be the most appropriate application of anthroponics for us. Even though we’re capable of growing highly productive leafy greens, we may not be capable of finding anyone to eat them. Pee-into-protein with anthroponics is a powerful story: one where humans and their waste become an integral part of a safe, and potentially highly productive, closed-loop nutrient cycle.
One final note: Those just entering the conversation about fertilizing with urine shouldn’t be intimidated by the complexity of hydroponic growing. Fertilizing plants and feeding the soil biome with urine is absolutely practical at the home garden scale. For more on soil applications of urine, please read part 1 of this series, watch our video series, and refer to posted references for more. As always, feel free to send comments by email.