In his posthumously published 1911 work Farmers of Forty Centuries, USDA agronomist F. H. King documents his travels studying the intensive agriculture of China, Korea and Japan. Multiple references are made to the efforts of farmers and city-dwellers alike to collect and distribute human waste to nearby farms as a valuable source of ongoing fertility:
“In such public places as railway stations provision is made for saving, not for wasting, and even along the country roads screens invite the traveler to stop, primarily for the profit of the owner, more than for personal convenience.”
Ancient Roman launderers known as fullones collected human urine from the public in small pots set alongside the street, diluted it with water and used it to aid in removing dirt from clothes.
In a vain attempt at producing gold, 17th century alchemist Hennig Brand accidentally discovered elemental phosphorus while heating in a retort the residues of some 5,500 liters of boiled down urine.
In his book At Home: A Short History of Private Life, author Bill Bryson writes about how before “sanitation’s greatest champion” Joseph Bazalgette started work on the central sewers of London, he had built his reputation on a plan to construct public urinals throughout the city, where then the urine would be collected and sold to industrial processors of alum.
Historically speaking, it’s clear that the current ‘flush and forget’ system of human waste disposal is abnormal.
In Bazalgette’s time, London’s growing population was experiencing cholera outbreaks that were widely thought to be the result of breathing the foul smelling air around the open sewers that led to the Thames river. It wasn’t yet widely understood that diseases were spread by fecal contamination in drinking water. Bazalgette’s contribution was to enclose sewers and pump and dump the waste further downstream, offsetting the offensive smell, while also incidentally beating the cholera crisis by moving the human waste further away from the source of London’s drinking water.
Nowadays, wastewater treatment systems are very effective at keeping fecal-derived pathogens out of our drinking water, but it comes at a cost. In a system where nutrient-rich liquid waste is diluted with flush water, mixed with fecal matter and whatever else people put down the drain, nutrient recovery at the sewage treatment plant becomes challenging and expensive. The majority of modern sewage plants are designed to remove most of these nutrients before discharge, typically either through chemical flocculation, various means of microbial immobilization or some combination of both. The resulting phosphorus and nitrogen-rich sludge may be anaerobically digested or further treated and applied as a (somewhat controversial) fertilizer, but more often than not (as is the case with my local wastewater plant) it is incinerated or landfilled. Moreover, although the liquid effluent from sewage plants is largely free of harmful pathogens, some contamination still persists: pharmaceutical contaminants or hormones may still be present, along with remaining nitrogen and phosphorus, further contributing to nutrient pollution in our waterways.
In recent decades, researchers in Europe and stateside organizations like the Rich Earth Institute in Vermont are advocating for a new approach to managing human waste where nutrient reclamation and sustainability takes center stage. Sometimes called Ecological Sanitation or ecosan, these are whole systems designs that attempt to ‘close the loop’ on human waste and agriculture. Common among these designs are urine diverting dry toilets (UDDT) where urine is kept separate from feces and flush water and stored in holding tanks to be applied directly as a fertilizer at nearby farms.
Urine diversion represents a sort of ‘low-hanging fruit’ in ecosan system designs. Compared to feces and kitchen waste water, urine is very easy to manage for pathogen reduction. By volume, urine accounts for only 1% of domestic wastewater while comprising up to 80% of the nitrogen, 55% of phosphorus and 60% of potassium. Urine is a potent fertilizer that is well balanced for grain production: an often cited Swedish publication from 2004 states that over the course of one year the average person produces enough urine to fertilize a 250kg crop of maize.
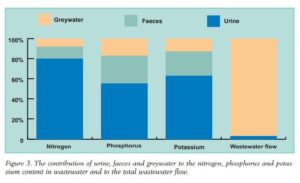
Urine accounts for most of the possible major plant nutrients that are flushed away in domestic wastewater.Source
Seeking a deeper understanding of the science of nutrient cycling, in early 2020 I set out to explore firsthand the historical and relevant modern uses for human urine. In a way it was good timing – the isolation brought on by the Covid-19 pandemic enabled me to invest more time in these admittedly anti-social projects. Ultimately, there’s too much to cover in one post. Here I’ll cover in detail the issues with urine collection and agricultural applications.
In later posts I’ll share my efforts with a historical method of using urine for processing indigo dyes and saltpeter production, experiments with the use of urine in recirculating hydroponic systems (known as anthroponics, or pee-ponics), and lastly I’ll share my trials with a low-tech process of struvite precipitation, yielding a potentially valuable, easily transported and stored urine-derived mineral fertilizer. But first, in order to discuss urine applications in any detail, we need to know more about what’s in it.
Composition
Healthy human urine is between 91% and 96% water. Over half of the non-water fraction is the nitrogen-containing compound urea. Nearly all of the Nitrogen in fresh urine is the form of urea. The remaining portion is a panoply of mostly innocuous organic compounds and inorganic salts.
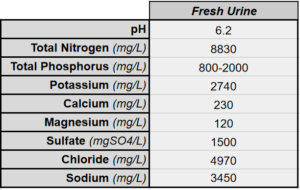
These figures for average nutrient concentration in human urine were published in the 2011 report: Technology Review for Urine Diversion Components
The elemental composition of urine changes with diet. Urea is a product of the metabolism of protein. Meat-eaters should expect their urine to have higher nitrogen levels, as well as higher levels of phosphorus and calcium. Conversely, those with a largely vegetarian or protein-limited diet should expect a higher pH urine with a higher ratio of potassium. Additionally, urine collected in the morning – the first after waking up – has been shown to have comparably higher levels of urea, calcium and phosphorus.
It has been said that adults are essentially at a ‘steady-state’; major plant nutrients nitrogen and phosphorus are no longer needed to grow, and thus not retained in the body. A diet rich in minerals will produce mineral-rich urine. On the flipside, diets high in common salt are going to reflect high sodium and chloride levels in urine. Pharmaceuticals can be persistent too, leading to valid concerns over the use of urine as a fertilizer and subsequent accumulation in agricultural soils; this has been the focus of much ongoing research, where a symbiotic application of biochar may be a solution. Urine is the product of filtering blood through the kidneys and contains only substances that have been metabolised. Heavy metals are largely a non-issue; metals are removed from the body as feces – a combination of both metablised and non-metabolised material. Interestingly it has been shown that when compared to expulsion via urine, sweating from the result of vigorous exercise is a much more effective means of removing metals from the body.
Is it Sterile?
Technically, no. Contrary to popular belief, human urine as it exits the body is not absolutely sterile. However, it should be noted very few diseases are transmitted via pathogens in healthy urine. In Carol Steinfeld’s book Liquid Gold: the Lore of and Logic of Using Urine to Grow Plants we are told on the authority the Swedish Institute of Infectious Disease Control that significant urine-transmitted diseases Leptospirosis and schistosomiasis are only of particular concern in humid, tropical environments. Salmonella is potentially transmittable through urine but is inactivated quickly after excretion.
There seems to be a general consensus among urine-reuse advocates that the risks of infection are nil if the urine is collected only from healthy members of your own household and destined for application on crops consumed only by the household. The conventional wisdom here is that you cannot get a disease from your own urine that you don’t already have. However, problems arise with fecal cross-contamination, and I suspect this is more often the case than I expect most people would like to know. In her presentation for the 2020 Rich Earth Summit, ASU researcher Daniella Saetta and team shared their work in identifying microbial communities in urine collected in waterless urinals – of all the samples, none were without contamination. For all practical purposes we should assume urine may harbor pathogenic bacteria and that come harvest time you may decide to share the produce with friends outside of the household. This is not to say we should be scared, but rather cautious. The risks can be easily mitigated through a regime of pre-treatment and best practices upon application in the garden.
Pre-Treatment for Safer Agricultural Use
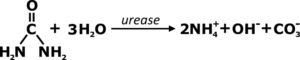
Recall that fresh urine is slightly acidic. It’s mostly water, while the remaining portion is mostly urea, along with some inorganic salts and various organic compounds. Among these organic compounds is the enzyme urease. Urease is naturally found in both urine and soils, and is ubiquitous among other many species of plants, fungi and bacteria. Urease is the catalyst by which through hydrolysis urea is ultimately converted to ammonium and hydroxide and carbonate ions.
Hydrolysis of urea and the resulting conversion will raise the pH from its starting point to at least 9. Pathogens are deactivated after a length of exposure in this high pH environment. This is an easy strategy for pre-treating urine prior to agricultural application – it’s largely a spontaneous process, all that is required is proper storage. Odorless ammonium easily shifts to gaseous ammonia, complete with it’s potent, distinctly pungent odor. Storage containers should remain closed but not necessarily gas-tight. The goal is to keep this volatile nitrogen dissolved in water, rather than lost to the air.
Under normal circumstances, hydrolysis may take anywhere from a few days to weeks. The speed of the reaction is largely temperature dependent – higher temperatures will speed up the reaction while very cold temperatures will nearly stop it. The rate of the reaction is also dependent on the amount of urease in the urine. Scientists have shown this through supplementation with urease found naturally concentrated in the jack bean. Researcher Henrique Sanchez has shown that by adding 1 gram of dehulled, crushed watermelon seeds to 100 mL fresh urine he was able to facilitate hydrolysis within 20 minutes.

Addition of watermelon seeds can speed up the hydrolysis reaction. In my trials I determined seeds should be crushed with a mortar and pestle, but dehulling was tedious and unnecessary. 24 seeds (.54g when dehulled) helped bring 600mL of 6.8pH urine up to 9 in less than 20 hours.
Hydrolysis of urine is an easy way to safeguard urine from pathogenic bacteria but it is not without its downsides. Containers must be kept at a moderate temperature for at least a month or more. The WHO recommends retention times above 8.8pH for 1 month when applied to fodder crops or those that will be later processed, and 6 months when applied on crops to be eaten raw.
The conversion of urea to volatile ammonia all but sure ensures that some nitrogen will be lost to the atmosphere. The smell is potent, and even though it has been said to dissipate quickly, make no mistake: doing anything with aged urine is likely going to be a lonely experience.
Another complication associated with hydrolyzed urine is the formation of struvite. Struvite is a mineral form of magnesium, ammonium and phosphate that precipitates out of stored urine as the pH rises. The formation of struvite leads to scaling of fixtures and clogging of pipes in urine diversion systems. The sediment builds up on the bottom of collection tanks and can clog fertigation equipment. It also changes the nutrient profile of the urine – all of the magnesium and most of the phosphorus will precipitate. Calcium too, will precipitate under high pH conditions. This is not to say these minerals are unavailable as plant nutrients – quite the contrary – struvite formation has been seen by some creative engineers not as a problem, but as a low-tech and accessible way to reclaim nutrients from urine in a solid form. Gardeners can be sure the mineral component of urine is delivered to soil through dilution and stirring before application.
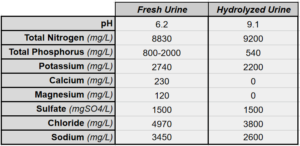
Nutrient Analysis of Human Urine Before and after Hydrolysis. Source
Problems associated with struvite and ammonia losses from hydrolyzed urine are one of the reasons researchers are looking for alternative ways to sanitize urine. Some have suggested treatment with citric acid to deactivate pathogens by adjusting pH down while also preventing struvite formation. The Rich Earth Institute employs a pasteurizing system. Others in the permaculture community have experimented with fermentation of urine along with wood ash and dynamic accumulators such as comfrey. Lacto-fermentation of the comparably phosphorus and calcium-rich morning urine is the method I’ve chosen for use in my recirculating hydroponic system (more on this in a future post).
Urine as a Fertilizer
Urine is a free and natural source of fast-acting, soluble nitrogen that compares well to widely-used industrially-produced urea and ammonium fertilizers. Recall that nearly all of the nitrogen in fresh urine is in the form of urea. Upon application in the soil, as in urine hydrolysis, soil-borne urease works quickly to convert urea nitrogen to ammonium nitrogen. Ammonium is in itself a plant-available source of nitrogen; ammonium may also be converted in the soil to plant-available nitrates by way of nitrifying bacteria. Nitrate conversion typically occurs in a few days. Biologically active soils will see faster conversion.
Other major plant nutrients – phosphorus, potassium and sulphur are found in urine in their readily available ionic form. Researchers in Sweden determined that locally-sourced urine had an NPK ratio of roughly 12:1:3 and was well-proportioned as a fertilizer for cereal production. Still, nitrogen is the primary nutrient here – urine alone is suited for nitrogen hungry leafy vegetables; supplemental potassium will be required to balance urine as an appropriate fertilizer for fruiting vegetable crops. Joe Hollis from Mountain Gardens mixes urine directly in wood ash – a potent source of potassium, calcium and micronutrients. Alkaline potassium and calcium will quickly raise the pH and deactivate potential pathogens, but will also encourage loss of nitrogen through ammonia gas formation. Mixing urine and ash can be a suitable way to create a balanced fertilizer that will be best stored in closed, corrosion resistant containers.
Depending on diet, urine is likely to have a concerning amount of sodium. Few common vegetable plants actually need any sodium at all, and therefore sodium accumulation in soils can reach harmful levels if left unchecked. In our humid climate in the Eastern United States rainfall will usually be enough to leach unwanted salts in outdoor gardens. However, gardeners must take special care not to allow excessive salt buildup anytime when growing under cover, or during periods of drought. This is especially the case with any indoor growing – potted plants, greenhouses for example. This is one reason why occasional deep irrigation is often recommended.
Application Methods and Rates
As a directly applied fertilizer, urine should be applied in methods that minimize ammonia volatilization, both for fertilizing efficiency and curbing offensive smells. Band application, low to the ground, is most appropriate for larger scale projects. In the garden, ammonia losses can be mitigated by applying in small holes or furrows alongside crops and immediately covered after application. The same technique can be applied with mulches when pulled aside and covered again after application. As a rule-of-thumb, keep urine 4” away from the base of the plant. Trees can be fertilized to the dripline. Nitrogen efficiency will be greatest in soils that are rich in organic matter, where urine is readily absorbed and won’t leach through or evaporate quickly – reducing losses from both above below.
Avoid spraying altogether – foliar application will damage leaves as water evaporates and concentrates salts. Application of through drip irrigation is likely to cause problems due to precipitation, but may be an appropriate method for the remaining phosphorus-free, yet still nitrogen-rich liquid that remains after struvite precipitation.
Dilution of urine with water prior to application is a common practice that isn’t entirely necessary. The Rich Earth Institute determined that there were no adverse effects of applying undiluted to hay. However, in the garden more precise application is possible when urine is diluted, and dilution helps prevent over-application and alleviates the risk of damaging roots. Garden vegetables are likely less tolerant of the high sodium content in urine; excess water in diluted urine will help evenly distribute all minerals, lessening the impact of concentrated salts. On a large scale dilution is not always practical, increasing labor and contributing to soil compaction issues, where larger tanks are required to carry additional water. A better strategy may be to apply urine at full-strength followed by light irrigation. In either case, urine is best not applied to dry soils.
Application rates are dependent on nitrogen needs of the crop at the time of application. Precise rates are all but impossible to determine without a urine nutrient analysis, however, we can make some assumptions based on published data. The Rich Earth Institute estimates there are .05 lbs of nitrogen (N) per gallon of urine. Much of the Swedish literature assumes 7 g N per liter – the equivalent of .063 lbs per gallon. Assuming .05 lb N/gal, then 20 gallons will provide 1 lb N over 1000ft² – for reference, this is the amount of nitrogen a typical suburban homeowner would apply two or three times a year to their front lawn. A typical corn N recommended application rate may be 240 lbs/acre, or 4800 gallons urine. Swedish trials found significant improvement in yield of winter wheat, assuming 7 g N/l, with an application rate of 120 Kg N/ha – the equivalent roughly 1,800 gallons urine. According to Swedish estimates the average person produces between about 1.5 and 2.5 liters urine a day – the equivalent of 145 to 241 gallons/year. Farm-scale urine fertilization clearly takes a community-scale collection effort. Such a project requires committed participation from a range of individuals – daunting, yes, but not impossible. Larger scale projects in Sweden have been implemented with installed urine diversion toilets and collection tanks and for their large-scale trials and the Rich Earth Institute has a program for accepting donations of urine from members of the local community.
Practical Advice
At a smaller scale the urine collected from even a single person can make an appreciable impact in the garden. Collecting a little over a quart a day, a single person can supply a ½ lb N every month for a 1000ft² garden. Vegetable crops are only fertilized during a portion of the growing season, so if urine is collected and stored throughout the year, one could reasonably fertilize a garden 3 or 4 times that size. Alternatively, urine can be used in ways that support fertility without direct application to the soil.
At home I keep a smaller dedicated vegetable garden space, a small orchard of 10 fruit trees and grapes, along with a patchwork of ramial chip mulched beds. I collect urine in a simple five gallon bucket in a private location outside on my property. Only 3 or so partially filled buckets will be stored and treated through hydrolysis for direct application on garden vegetables.
Lately I’ve been collecting morning urine separately in quart or gallon size plastic jugs and fermenting it and storing for a month before use as the primary nutrient for my recirculating hydroponic system – a little goes a long way here. Smaller storage jugs are easier to measure, dilute and pour into handheld watering cans for targeted application. As a male, it’s easy to use these, and for now, I prefer to keep it simple as I don’t expect anyone else in my family will be excited about contributing their own for a long time.
Outside of the garden, urine is applied in the orchard and occasionally on the compost pile. Diluted urine is applied once a year in the early spring on select trees in the orchard. Often I’ll take a half-full 5-gallon bucket and top it off with water before pouring over a thick layer of ramial wood chip mulch under the drip line of individual trees. Nearly all of my fresh food waste goes to the worm bins – urine is not welcome here. However, in the fall I’ll start a passively aerated compost pile for carbon-rich yard and garden cleanup, and here, urine is absolutely a helpful way to apply nitrogen that facilitates decomposition of woody material.
It seems in nearly all of our biochar presentations, someone in the crowd inevitably mentions charging biochar with urine. There is wisdom here. High temperature biochars especially have been shown to retain nitrogen and phosphorus anions. A common strategy here is to pre-treat, or ‘charge’ the biochar with urine before mixing as an ingredient in the compost. I use this method as means of including both nitrogen and biochar in my carbon-rich static, passively aerated piles. In conventional 30:1 C:N thermophilic compost,, it stands to reason that biochar may be more effective at absorbing ammonia gases when applied without a urine pre-treatment.
Conjecture
It was about this time last year I set out to begin establishing a dye garden. Dyeing with indigo pigment is a fascinating process involving reduction in an alkaline vat – a condition that historically was met by using aged urine. Out of some morbid curiosity of all things tedious, groomed through last year’s work with wood ashes, I set out to attempt the process on my own. Again, I’ll speak more about this in a follow-up post.
Germination of my Japanese Indigo (Persicaria tinctoria) was very successful – 90 frost-tender plants were ready for transplant in the greenhouses by mid-march. Started early enough and managed carefully, some growers can expect 3, sometimes 4 harvests throughout the growing season. However, it was about this time the Covid-19 pandemic became more severe and indigo plants were no longer a priority – space in the greenhouse was to be reserved for food and medicine.
Mid-April is our average last frost. In prior years our final spring frost had come very early and I retained some hope I could transplant by the first of April in my own garden. Around 60 plants were well established after a month in a 40 ft² before a late April cold snap arrived. A low tunnel kept enough heat in to prevent frost damage, but the cold was enough to signal early flowering.
Nitrogen is known to encourage vegetative growth; urine is a well matched fertilizer for leafy greens. Following this logic, I pinched off early flowers and applied a heavy dose of 1 gallon urine, diluted with 3 gallons of water, over the 40 ft² bed. In two-week intervals I repeated this process 3 more times so that an estimated .2lbs of N was applied in an 8-week period. By late spring flowering had largely stopped, and despite the early season setback, I was still able to harvest completely 3 times throughout the growing season. Urine would prove to be well-suited for both growing and processing my indigo crop.

Did applying a heavy application of urine fertilizer force my early-flowering Japanese Indigo plants back into vegetative state?
Helpful References
The Rich Earth Institute is researching and advocating for urine use in agriculture in the United States. Their Home Use Guide is a helpful summary of best practices in the garden.
Guidelines on the Use of Urine and Feces in Crop Production
A Technology Review of Urine Diversion Components
A report on urine diversion with concrete examples of use in agriculture: Urine Separation – Closing the Nutrient Cycle
WHO Report: Urine Diversion – Hygienic Risks and Microbial Guidelines for Reuse