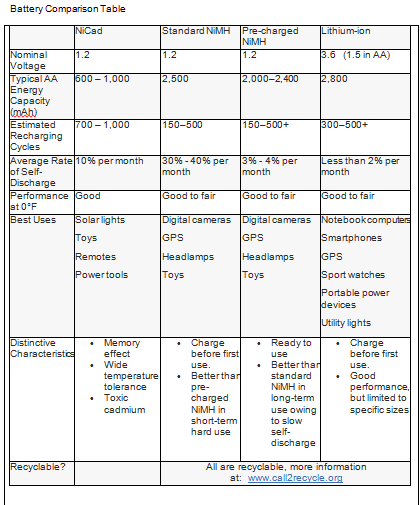
Batteries have become such a ubiquitous part of life that we often don’t give them much thought until they fail. At that point, they’re an inconvenience, an unwanted expense, and to most of us a profound mystery. Yet they’ve brought so much accessibility to our lives that it would be difficult to imagine getting by without them.
Cell phones, power tools, laptops and tablets, medical and communications equipment, portable devices, and even vehicles all rely on some form of battery storage. And since batteries span a full spectrum of applications, chemistries, performance, and environmental impact, it can be difficult for a consumer to make a good—not simply convenient—decision when making a purchase.
The common dry-cell battery found its way to market in 1896 and used a zinc-carbon and paste construction to replace earlier liquid solutions, opening the door to portable electric devices, most notably the flashlight, since it would work in any orientation. That first cell was essentially the plain-vanilla D-cell 1.5-volt general purpose battery still used today. A single-use disposable, it served nearly a century no questions asked until we began to question the very fabric of our throw-away culture.

Curiously enough, several years after the National Carbon Company (predecessor of Eveready/Energizer) introduced the dry cell, a rechargeable Nickel-Cadmium (NiCad) alkaline battery was developed in Europe. Alas, it was a wet cell and relatively expensive, and so the technology didn’t find its way to U.S. shores until after the Second World War.
Following several decades of persistent research into alkalines, technological breakthroughs occurred beginning in the 1980’s when Lithium-Ion (Li-ion) and Nickel Metal Hydride (NiMH) chemistries were developed for satellites and specialty applications. Today, there is an impressive range of battery chemistries and constructs, seven in the Lithium family alone. But for the purpose of this blog, I’m going to focus on the most popular consumer rechargeables and single-use batteries common to specific devices because that’s what most people use.
What’s in a Name?
Let’s begin with terminology. What we commonly call a battery is actually a cell, or a single electrochemical unit that converts chemical energy into electrical energy. When two or more cells are assembled together, it becomes a battery, or group of cells.
The batteries that we’d typically use in our devices have positive and negative terminals and two internal components called electrodes—one cathode which carries a positive charge, and one anode to carry a negative charge. These are embedded in an electrolyte, a material that conducts electrons between the terminals. When the battery is installed and the device is turned on, an oxidation reaction takes place and electrons move through the electrolyte to exit the anode and power the device.
Because they’re often called storage batteries, there’s a misconception that batteries and cells “store” electricity. What actually happens is that they store energy in a chemical form, and when a conductor provides a path for electrons to move and do work, the process initiates the chemical reaction. In time, the reaction degrades the chemicals and they eventually stop supplying electrons. In a rechargeable battery, the electrons’ direction of flow is reversed during charging, reversing the electrochemical process and restoring the electrodes to their original chemical state.
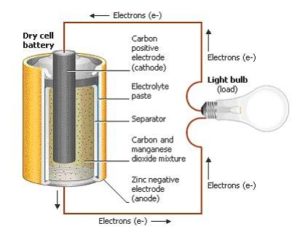
Primary batteries are single-use disposable units, the AAA, AA, C, D, 9V, and button/coin types familiar to us in many products from flashlights to cameras. These are by far alkaline or lithium batteries, though general-purpose zinc-carbon cells are still manufactured.
Secondary batteries are rechargeable and are usually device-specific, though secondary letter-size and 9V batteries that use NiMH and Li-Ion chemistries are widely available. (The most popular Li-ion cells are 3.6V but 1.5 volt cylinder sizes exist.) They have largely replaced reusable alkaline batteries, a transition technology developed in the early 1990’s as a green option to single-use disposables.
Other than determining by shape and size that a battery will actually fit in your device, there are other things to be aware of when battery shopping. Voltage output is indicated on the cell. So an AA configuration not only tells you that the cell is 14.5mm x 50mm cylinder, but that it has a nominal voltage of 1.5V. Coin and button-cell batteries use an alpha-numeric code, where the first two letters indicate the chemical composition and the shape, followed by four numbers indicating the size in millimeters, diameter by height.
Nominal simply means in name only. Actual voltage of an AA alkaline battery fresh out of the pack is about 1.6 volts. A reusable alkaline of the same size, 1.2 volts. And an AA rechargeable NiMH between 1.2 and 1.25 volts. So how do they all work in the same device? Because the voltage of a single-use alkaline cell declines immediately as the battery discharges, putting it on par with the reusable and the NiMH, which tend to maintain their consistent voltage much longer under use.
Battery capacity is measured in milliamp-hours (mAh) and may range from 900 mAh to over 2500 mAh depending on chemistry. A battery with a rating of 2000 mAh should deliver a current of 2000 milliamps for one hour. Though higher energy capacity batteries will generally power a device longer, high-drain devices like digital cameras will deplete an alkaline cell more quickly than it will a NiMH cell because of their high rate of draw. In comparison, the Nickel Metal Hydride cell can tolerate higher rates without exhausting the battery.
Self-discharge rate is an important factor for devices that spend a lot of time waiting for use, such as a flashlight or smoke alarm. It’s also a measure of shelf life, as when stocking up for later emergency use. Power loss on standby can be fairly high in standard NiMH batteries—up to 40% capacity in a month. Hybrid NiMH batteries (marketed as pre-charged cells) do much better in this category, and Li-ion chemistries excel, along with standard alkaline batteries.
Charging cycles refers to how many times a battery can be charged and discharged before its useful life is over. Alkaline batteries are generally not rechargeable (except for the reusable types) but NiMH cells can be cycled over 500 times and some Li-ion cells over 1000. Pre-charged NiMH batteries perform slightly better than standard NiMH cells.
Single-use batteries may be less costly initially but they take an environmental toll over time in that they cannot be conveniently recycled and are usually just discarded. Occasionally local recycling facilities may sponsor a hazardous household waste collection event where they can be turned in, or you can take advantage of the mail-in or take-back programs offered.
Rechargeable batteries are a better choice in that they are generally recyclable. NiCad cells are hazardous waste and must be recycled when they are spent. NiMH and Li-ion laptop batteries are non-hazardous, but can be recycled through local programs or through Call2Recycle outlets. Some button-cell batteries contain silver and mercury, but many are mercury-free and relatively benign. Regardless, these too should finish their lives in a recycling facility.
One aspect of battery manufacture that doesn’t always get the attention it warrants is the necessary extraction of conflict minerals. Mining and mineral harvest are part of any manufacturing process, but the evolution of battery technology has increased the demand for materials such as lithium and graphite, and with certain chemistry types, rare metals such as cobalt, nickel, and manganese. These are often mined and processed in developing and politically unstable nations, where worker rights and protections are meager at best. Certainly something to keep in mind when buying batteries, and certainly an incentive to maintain them properly to extend their useful life.

Choosing a Battery
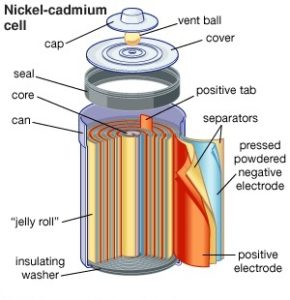
If this were a discussion on batteries for electric vehicle design, factors like weight, size, and safety would be key considerations. Since those factors are already established in a laptop or flashlight or any other consumer device, a buyer should be comparing cost, capacity, charging cycles, and disposal in order to get the best value. NiCad cells, for example, have largely been replaced by NiMH cylindrical batteries because they don’t contain highly toxic cadmium and have a 40% greater energy capacity.
In most cases, if the other parameters are satisfied, contrasting cost to cycle life offers a good yardstick for value comparison. For example a popular pre-charged NiMH 800mAh AAA cell is available for $10.49 a 4-pack, or $2.62 per unit. Another manufacturer sells standard NiMH cells with the same capacity for $2.08 per unit. Even though the cost of the pre-charged is $0.54 higher, their cycle life is nearly three times greater so they represent a better value over time.
Charging Strategies
Proper charging and discharging practices are an essential part of maintaining the life expectancy of any type of battery. In normal use, a battery performs best when it’s not exposed to extremes of heat or cold, especially sub-zero temperatures. If you can schedule charging sessions once the pack has been acclimated to room temperatures, it’s better for battery health.
When NiCad batteries were more common, users were cautioned against the “memory effect,” or recharging after shallow discharges, which encourages that chemistry to absorb only the amount of energy it supplied during its previous discharge. NiCad’s, in fact, require a periodic full discharge and prefer a fast charge and immediate use to being cradled in the charger for days at a time.
NiMH batteries, and Li-ion’s particularly, share no such memory quirks. They can be recharged at any point in their cycle with no recall effects. They will, however, last considerably longer if they are discharged within the shallow end of their range before recharging. A good rule of thumb is a 30% discharge, with 50% acceptable. Deep discharges, especially under high loads, can reduce cycle life by half. Charge times for NiCad batteries can be as quick as one hour. The others need 2 to 4 hours to full charge, with slightly less time for reusable alkalines.
The slab, block, prismatic, and pack style batteries found in cell phones, laptops, and other consumer devices are often Lithium-ion chemistries and come with dedicated chargers. Rechargeable cylinder style cells are similarly charged, or can be used in what’s known as an intelligent digital balance charger which accepts NiCad, NiMH, or Li-ion cells (up to 20,000 mAh) and automatically adjusts voltage and charge rate to suit the chemistry of the battery.

When charging multiple batteries, avoid mixing different capacities and different brands, and try to maintain the same age cells in a simultaneous charge. The same practice should apply when using the batteries as well; consistency promotes better performance and longevity.
There is also a wide array of solar chargers on the market that provide a reliable source of power whether you have access to the grid or not. Compact windowsill chargers are made to supply energy to multiple configurations of NiMH and NiCad cells, and similar packages are available for older style reusable alkaline batteries too.

Borrowing from the outdoor recreation venue, foldable portable chargers provide greater wattage and work with many devices including cell phones, rechargeable lanterns, tablets, digital cameras and the like. More robust configurations with adapters will charge a variety of larger devices including compact power stations safely, with an assortment of voltages.

Reliable battery technology has brought us to a place unheard of even a few decades ago. Using it wisely is the responsibility of all of us if we’re to be educated consumers.